Liquid Propellant Rocket Engines Pdf
Posted in HomeBy adminOn 02/11/17Liquid rocket propellant Wikipedia. The highest specific impulse chemical rockets use liquid propellants liquid propellant rockets. They can consist of a single chemical a monopropellant or a mix of two chemicals, called bipropellants. Bipropellants can further be divided into two categories hypergolic propellants, which ignite when the fuel and oxidizer make contact, and non hypergolic propellants which require an ignition source. DescriptioneditAbout 1. In the U. S. alone at least 2. No completely new propellant has been used for nearly 3. Here is your handydandy cheatsheet of rocket engines. Use this as a jumpingoff point, there is no way I can keep this uptodate. Google is your friend


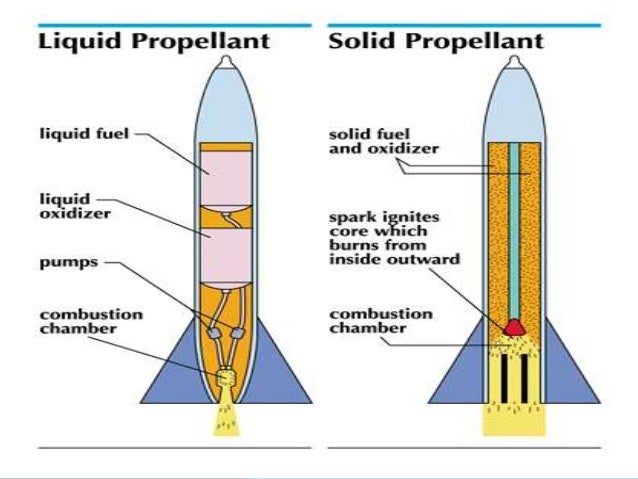 North American Rocketdyne Boeing Rocketdyne Space Engines 1955 2005 Updated 6 April 2014. Rocket Apparatus for Inventors and Experimenters motors, hardware, electronics, test stand equipment, literature, graphite and much more A liquidpropellant rocket or liquid rocket is a rocket engine that uses liquid propellants. Liquids are desirable because their reasonably high density allows the. NAA 75110. The NAA 75110 rocket engine is a liquid propellant, turbopump fed, single thrust chamber unit, which is rated at a sea level thrust of 75,000 lb for a. StagingandControlofRocketsandMissiles_A7A8/clip_image008.jpg' alt='Liquid Propellant Rocket Engines Pdf Free' title='Liquid Propellant Rocket Engines Pdf Free' />Many factors go into choosing a propellant for a liquid propellant rocket engine. The primary factors include ease of operation, cost, hazardsenvironment and performance. They can consist of a single chemical, a monopropellant, or two, called bipropellants or other mixtures. Bipropellants can be either hypergolic propellant or nonhypergolic. A hypergolic combination of oxidizer and fuel will start to burn upon contact. A nonhypergolic needs an ignition source. HistoryeditEarly development, 1. Robert H. Goddard on March 1. On March 1. 6, 1. Robert H. Goddard used liquid oxygen LOX and gasoline as rocket fuels for his first partially successful liquid propellant rocket launch. Both propellants are readily available, cheap and highly energetic. Oxygen is a moderate cryogen as air will not liquefy against a liquid oxygen tank, so it is possible to store LOX briefly in a rocket without excessive insulation. World War II eraeditGermany had very active rocket development before and during World War II, both for the strategic V 2 rocket and other missiles. The V 2 used an alcoholLOX liquid propellant engine, with hydrogen peroxide to drive the fuel pumps. The alcohol was mixed with water for engine cooling. Both Germany and the United States developed reusable liquid propellant rocket engines that used a storeable liquid oxidizer with much greater density than LOX and a liquid fuel that would ignite spontaneously on contact with the high density oxidizer. The German engine was powered by hydrogen peroxide and a fuel mixture of hydrazine hydrate and methyl alcohol. The U. S. engine was powered by nitric acid oxidizer and aniline. Both engines were used to power aircraft, the Me 1. B Komet interceptor in the case of the German engine and RATO units to assist take off of aircraft in the case of the U. S. engine. 1. 95. During the 1. 95. Large strategic missiles need to sit in land based or submarine based silos for many years, able to launch at a moments notice. Propellants requiring continuous refrigeration, which cause their rockets to grow ever thicker blankets of ice, were not practical. As the military was willing to handle and use hazardous materials, a great number of dangerous chemicals were brewed up in large batches, most of which wound up being deemed unsuitable for operational systems. In the case of nitric acid, the acid itself HNO3 was unstable, and corroded most metals, making it difficult to store. The addition of a modest amount of nitrogen tetroxide, N2. Liquid Propellant Rocket Engines Pdf CreatorO4, turned the mixture red and kept it from changing composition, but left the problem that nitric acid corrodes containers it is placed in, releasing gases that can build up pressure in the process. The breakthrough was the addition of a little hydrogen fluoride HF, which forms a self sealing metal fluoride on the interior of tank walls that Inhibited Red Fuming Nitric Acid. This made IRFNA storeable. Propellant combinations based on IRFNA or pure N2. O4 as oxidizer and kerosene or hypergolic self igniting aniline, hydrazine or unsymmetrical dimethylhydrazine UDMH as fuel were then adopted in the United States and the Soviet Union for use in strategic and tactical missiles. The self igniting storeable liquid bi propellants have somewhat lower specific impulse than LOXkerosene but have higher density so a greater mass of propellant can be placed in the same sized tanks. Gasoline was replaced by different hydrocarbon fuels, for example RP 1 a highly refined grade of kerosene. This combination is quite practical for rockets that need not be stored. As of 2. 01. 7update, it is used in the first stages of many orbital launchers. KeroseneeditThe V 1 and V 2 rockets developed by Nazi Germany used LOX and ethyl alcohol. One of the main advantages of alcohol was its water content which provided cooling in larger rocket engines. Petroleum based fuels offered more power than alcohol, but standard gasoline and kerosene left too much silt and combustion by products that could clog engine plumbing. In addition they lacked the cooling properties of ethyl alcohol. During the early 1. US was assigned the task of formulating an improved petroleum based rocket propellant which would not leave residue behind and also ensure that the engines would remain cool. The result was RP 1, the specifications of which were finalized by 1. A highly refined form of jet fuel, RP 1 burned much more cleanly than conventional petroleum fuels and also posed less of a danger to ground personnel from explosive vapors. It became the propellant for most of the early American rockets and ballistic missiles such as the Atlas, Titan I, and Thor. The Soviets quickly adopted RP 1 for their R 7 missile, but the majority of Soviet launch vehicles ultimately used storable hypergolic propellants. HydrogeneditHydrogen gas is commercially produced by the fuel rich burning of natural gas. Carbon forms a stronger bond with oxygen so the gaseous hydrogen is left behind. Liquid hydrogen is stored and transported without boil off, because helium, which has a lower boiling point than hydrogen, acts as cooling refrigerant. Only when hydrogen is loaded on a launch vehicle, where no refrigeration exists, it vents to the atmosphere. Many early rocket theorists believed that hydrogen would be a marvelous propellant, since it gives the highest specific impulse. It is also considered the cleanest when used with a liquid oxygen oxidizer because the only by product is water. Hydrogen in any state is very bulky for lightweight vehicles it is typically stored as a deeply cryogenic liquid, a technique mastered in the early 1. Los Alamos. citation needed In the late 1. Centaur and Saturn upper stages. Even as a liquid, hydrogen has low density, requiring large tanks and pumps, and the extreme cold requires tank insulation. This extra weight reduces the mass fraction of the stage or requires extraordinary measures such as pressure stabilization of the tanks to reduce weight. Pressure stabilized tanks support most of the loads with internal pressure rather than with solid structures. The Soviet rocket program, in part due to a lack of technical capabilities, did not use LH2 as a propellant until the 1. Energiya core stage. Comparison to keroseneeditLaunch pad fires due to spilled kerosene are more damaging than hydrogen fires, primarily for two reasons. First, kerosene burns about 2. The second reason is its buoyancy. Since hydrogen is a deep cryogen it boils quickly and rises due to its very low density as a gas. Gtr2 Road Car Mod Download. Even when hydrogen burns, the gaseous H2. Pes 2013 Bundesliga Option File Ps3 there. O that is formed has a molecular weight of only 1. Kerosene on the other hand falls to the ground and burns for hours when spilled in large quantities, unavoidably causing extensive heat damage that requires time consuming repairs and rebuilding.
North American Rocketdyne Boeing Rocketdyne Space Engines 1955 2005 Updated 6 April 2014. Rocket Apparatus for Inventors and Experimenters motors, hardware, electronics, test stand equipment, literature, graphite and much more A liquidpropellant rocket or liquid rocket is a rocket engine that uses liquid propellants. Liquids are desirable because their reasonably high density allows the. NAA 75110. The NAA 75110 rocket engine is a liquid propellant, turbopump fed, single thrust chamber unit, which is rated at a sea level thrust of 75,000 lb for a. StagingandControlofRocketsandMissiles_A7A8/clip_image008.jpg' alt='Liquid Propellant Rocket Engines Pdf Free' title='Liquid Propellant Rocket Engines Pdf Free' />Many factors go into choosing a propellant for a liquid propellant rocket engine. The primary factors include ease of operation, cost, hazardsenvironment and performance. They can consist of a single chemical, a monopropellant, or two, called bipropellants or other mixtures. Bipropellants can be either hypergolic propellant or nonhypergolic. A hypergolic combination of oxidizer and fuel will start to burn upon contact. A nonhypergolic needs an ignition source. HistoryeditEarly development, 1. Robert H. Goddard on March 1. On March 1. 6, 1. Robert H. Goddard used liquid oxygen LOX and gasoline as rocket fuels for his first partially successful liquid propellant rocket launch. Both propellants are readily available, cheap and highly energetic. Oxygen is a moderate cryogen as air will not liquefy against a liquid oxygen tank, so it is possible to store LOX briefly in a rocket without excessive insulation. World War II eraeditGermany had very active rocket development before and during World War II, both for the strategic V 2 rocket and other missiles. The V 2 used an alcoholLOX liquid propellant engine, with hydrogen peroxide to drive the fuel pumps. The alcohol was mixed with water for engine cooling. Both Germany and the United States developed reusable liquid propellant rocket engines that used a storeable liquid oxidizer with much greater density than LOX and a liquid fuel that would ignite spontaneously on contact with the high density oxidizer. The German engine was powered by hydrogen peroxide and a fuel mixture of hydrazine hydrate and methyl alcohol. The U. S. engine was powered by nitric acid oxidizer and aniline. Both engines were used to power aircraft, the Me 1. B Komet interceptor in the case of the German engine and RATO units to assist take off of aircraft in the case of the U. S. engine. 1. 95. During the 1. 95. Large strategic missiles need to sit in land based or submarine based silos for many years, able to launch at a moments notice. Propellants requiring continuous refrigeration, which cause their rockets to grow ever thicker blankets of ice, were not practical. As the military was willing to handle and use hazardous materials, a great number of dangerous chemicals were brewed up in large batches, most of which wound up being deemed unsuitable for operational systems. In the case of nitric acid, the acid itself HNO3 was unstable, and corroded most metals, making it difficult to store. The addition of a modest amount of nitrogen tetroxide, N2. Liquid Propellant Rocket Engines Pdf CreatorO4, turned the mixture red and kept it from changing composition, but left the problem that nitric acid corrodes containers it is placed in, releasing gases that can build up pressure in the process. The breakthrough was the addition of a little hydrogen fluoride HF, which forms a self sealing metal fluoride on the interior of tank walls that Inhibited Red Fuming Nitric Acid. This made IRFNA storeable. Propellant combinations based on IRFNA or pure N2. O4 as oxidizer and kerosene or hypergolic self igniting aniline, hydrazine or unsymmetrical dimethylhydrazine UDMH as fuel were then adopted in the United States and the Soviet Union for use in strategic and tactical missiles. The self igniting storeable liquid bi propellants have somewhat lower specific impulse than LOXkerosene but have higher density so a greater mass of propellant can be placed in the same sized tanks. Gasoline was replaced by different hydrocarbon fuels, for example RP 1 a highly refined grade of kerosene. This combination is quite practical for rockets that need not be stored. As of 2. 01. 7update, it is used in the first stages of many orbital launchers. KeroseneeditThe V 1 and V 2 rockets developed by Nazi Germany used LOX and ethyl alcohol. One of the main advantages of alcohol was its water content which provided cooling in larger rocket engines. Petroleum based fuels offered more power than alcohol, but standard gasoline and kerosene left too much silt and combustion by products that could clog engine plumbing. In addition they lacked the cooling properties of ethyl alcohol. During the early 1. US was assigned the task of formulating an improved petroleum based rocket propellant which would not leave residue behind and also ensure that the engines would remain cool. The result was RP 1, the specifications of which were finalized by 1. A highly refined form of jet fuel, RP 1 burned much more cleanly than conventional petroleum fuels and also posed less of a danger to ground personnel from explosive vapors. It became the propellant for most of the early American rockets and ballistic missiles such as the Atlas, Titan I, and Thor. The Soviets quickly adopted RP 1 for their R 7 missile, but the majority of Soviet launch vehicles ultimately used storable hypergolic propellants. HydrogeneditHydrogen gas is commercially produced by the fuel rich burning of natural gas. Carbon forms a stronger bond with oxygen so the gaseous hydrogen is left behind. Liquid hydrogen is stored and transported without boil off, because helium, which has a lower boiling point than hydrogen, acts as cooling refrigerant. Only when hydrogen is loaded on a launch vehicle, where no refrigeration exists, it vents to the atmosphere. Many early rocket theorists believed that hydrogen would be a marvelous propellant, since it gives the highest specific impulse. It is also considered the cleanest when used with a liquid oxygen oxidizer because the only by product is water. Hydrogen in any state is very bulky for lightweight vehicles it is typically stored as a deeply cryogenic liquid, a technique mastered in the early 1. Los Alamos. citation needed In the late 1. Centaur and Saturn upper stages. Even as a liquid, hydrogen has low density, requiring large tanks and pumps, and the extreme cold requires tank insulation. This extra weight reduces the mass fraction of the stage or requires extraordinary measures such as pressure stabilization of the tanks to reduce weight. Pressure stabilized tanks support most of the loads with internal pressure rather than with solid structures. The Soviet rocket program, in part due to a lack of technical capabilities, did not use LH2 as a propellant until the 1. Energiya core stage. Comparison to keroseneeditLaunch pad fires due to spilled kerosene are more damaging than hydrogen fires, primarily for two reasons. First, kerosene burns about 2. The second reason is its buoyancy. Since hydrogen is a deep cryogen it boils quickly and rises due to its very low density as a gas. Gtr2 Road Car Mod Download. Even when hydrogen burns, the gaseous H2. Pes 2013 Bundesliga Option File Ps3 there. O that is formed has a molecular weight of only 1. Kerosene on the other hand falls to the ground and burns for hours when spilled in large quantities, unavoidably causing extensive heat damage that requires time consuming repairs and rebuilding.